When you pick up a prescription for insulin or a biologic drug like Humira, you might not realize your pharmacist could swap it for a cheaper version without telling you. That’s only possible if the biosimilar has been labeled interchangeable by the FDA. This isn’t just a marketing term-it’s a legal status with real consequences for your treatment, your wallet, and how your pharmacy operates.
What Makes a Biosimilar ‘Interchangeable’?
Not all biosimilars are the same. A regular biosimilar is a copy of a brand-name biologic drug-like Humira or Enbrel-that’s highly similar in structure and function. But to be called interchangeable, it has to pass a tougher test. The FDA requires switching studies where patients are moved back and forth between the original drug and the biosimilar, sometimes multiple times. The goal? Prove that switching doesn’t raise safety risks or reduce effectiveness.This is where biosimilars differ from regular generics. A generic pill, like lisinopril, is chemically identical to its brand-name version. You can swap it anytime. Biosimilars, though, are made from living cells-yeast, bacteria, or animal cells-and tiny changes in manufacturing can affect how they work. That’s why the FDA demands extra proof before allowing automatic substitution.
As of November 2023, the FDA has approved 41 biosimilars. Only 10 of them carry the interchangeable label. The first was Semglee, an insulin glargine product approved in July 2021. Then in August 2023, Cyltezo became the first interchangeable biosimilar for adalimumab, the active ingredient in Humira. These aren’t just theoretical-they’re already changing how patients get treated.
How U.S. Rules Are Different from the Rest of the World
The United States is the only country with a formal, nationwide system that lets pharmacists swap interchangeable biosimilars without checking with the doctor first. In Europe, Canada, and Japan, substitution usually requires the prescriber’s input or isn’t allowed at all. The European Medicines Agency doesn’t even use the term “interchangeable.”This U.S. approach was created by the Biologics Price Competition and Innovation Act (BPCIA) of 2009, part of the Affordable Care Act. The idea was simple: if patients can get the same results from a cheaper version, why not let pharmacies switch automatically to cut costs?
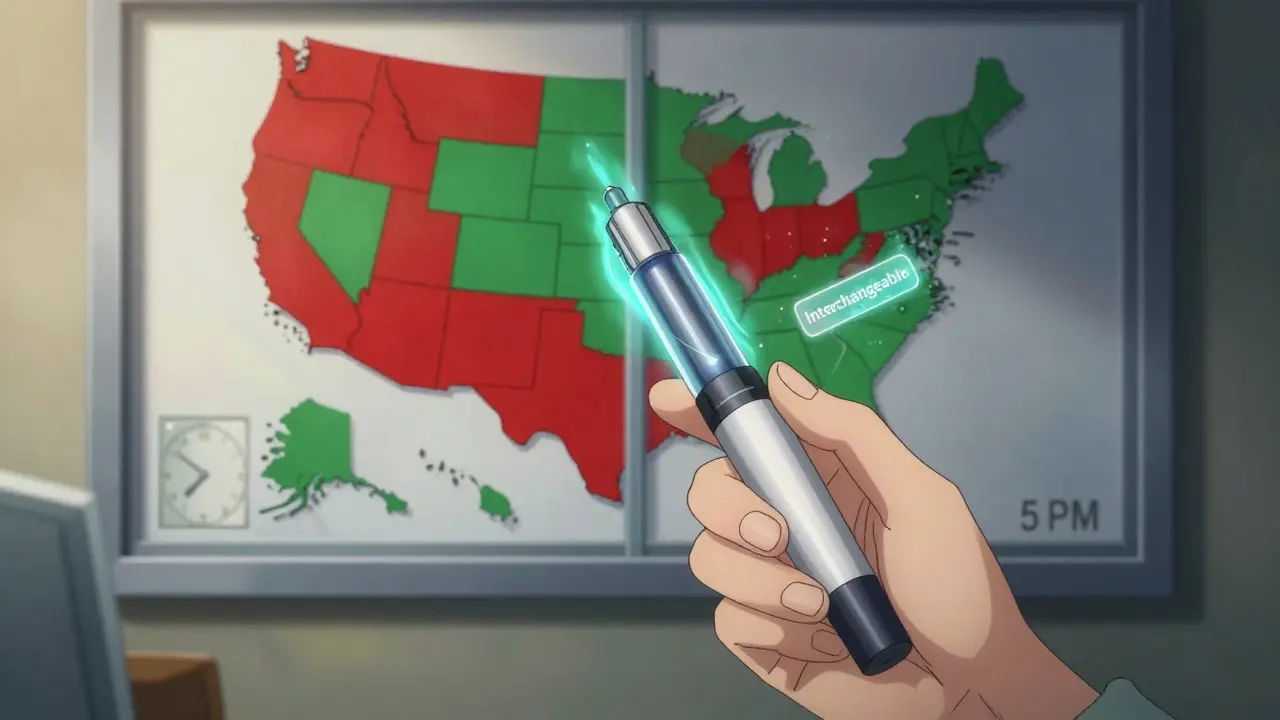
But the system isn’t uniform. While the FDA sets the federal standard, each state gets to decide how it’s applied. Forty states let pharmacists substitute interchangeable biosimilars without asking the doctor. Six states and Washington D.C. only allow it if the substitution saves the patient money. Four states-Alabama, Indiana, South Carolina, and Washington-require the prescriber to approve the switch every time.
Why This Matters for Patients
For many, interchangeable biosimilars mean big savings. Semglee, the interchangeable insulin, costs about 30% less than the original Lantus. Patients report saving $500 to $800 per month after switching. That’s life-changing for people who need insulin every day.But not everyone has a smooth experience. Some patients report no issues after switching. Others notice side effects-or worse, don’t know they were switched at all. One patient on a psoriasis forum said their pharmacy substituted Hadlima for Humira without notice, and they had an allergic reaction to an excipient in the new product. That’s a real risk. Biosimilars may have different inactive ingredients, and those can trigger reactions in sensitive people.
A 2022 survey by the National Psoriasis Foundation found that 63% of patients were happy with their biosimilar, but 28% were worried they weren’t told about the change. Transparency matters. In states like Arizona, pharmacists must notify patients in writing, record the product used, and send a copy to the doctor within five days. But in other states? No such requirement.
What Pharmacists Are Up Against
Pharmacists are caught in the middle. They have to know which biosimilars are interchangeable, which states allow substitution, and whether the patient’s insurance requires it. A 2022 survey by the National Community Pharmacists Association found that 67% of independent pharmacists were confused about state laws. One pharmacist on Reddit said, “In California, I have to check if it’s lower cost, but in Arizona I don’t. My system doesn’t differentiate between states.”Insurance plans add another layer. About 78% of commercial health plans automatically require substitution when state law allows it. That means even if you’re fine on your current drug, your insurer might push you to switch to cut costs. You can block it-if your doctor writes “dispense as written” on the prescription or uses a DAW code-but many patients don’t know they have that right.
To help, the American Pharmacists Association now offers a 2.5-hour online certificate course on biosimilars. Over 12,000 pharmacists have taken it as of late 2023. Still, the learning curve is steep. Pharmacists spend nearly 9 hours a year just keeping up with changing rules.
Market Impact and Future Outlook
The numbers show interchangeability is working. In the insulin market, Semglee captured 17.3% of the market within six months. Non-interchangeable biosimilars took nearly twice as long to reach that level. States with automatic substitution laws saw 18.7% higher biosimilar use for insulin compared to states without them.By 2026, biosimilars are expected to take 47% of the $168 billion biologics market. But that growth depends on resolving the current patchwork of state laws. Right now, a national pharmacy chain has to manage 50 different rulebooks. That’s inefficient and risky.
There’s also a political fight brewing. The Biosimilar Red Tape Elimination Act, introduced in 2022, wants to scrap the switching studies entirely and make every FDA-approved biosimilar automatically interchangeable. The drug industry (PhRMA) says that’s dangerous-patients could be switched multiple times without proper monitoring. The Biosimilars Council argues it’s unnecessary red tape that slows down savings.
The FDA’s stance? All approved biosimilars are safe and effective. Interchangeability doesn’t mean “better”-it just means you can swap it without calling the doctor. As FDA official Dr. Sarah Yim said in 2023: “Interchangeables are not a higher level of biosimilar.”
What You Should Do
If you’re on a biologic drug and your pharmacist switches you to a biosimilar:- Ask: “Is this interchangeable?”
- Ask: “Was this a substitution, or did my doctor choose it?”
- Check the label for the manufacturer name-different companies make different versions.
- If you feel worse, tell your doctor immediately. It could be the drug-or the excipients.
- If you want to stay on your current drug, ask your doctor to write “dispense as written” on the prescription.
Don’t assume a cheaper version is automatically right for you. Biosimilars are safe-but automatic substitution doesn’t mean automatic safety for every individual. Your body reacts to small changes. Know your options. Speak up. Keep track.
How This Affects the Future of Treatment
The big picture? Interchangeability is meant to lower costs and expand access. Biologics are expensive-some cost over $20,000 a year. Without competition, prices stay high. Biosimilars bring that down. But if patients lose trust because they’re switched without warning, or if adverse reactions go unreported, uptake will stall.The real challenge isn’t science. It’s systems. We have the science. We have the drugs. What’s missing is a clear, consistent, patient-centered way to use them. Until states and insurers align on rules, and until patients are truly informed, interchangeability will remain a tool with huge potential-and serious risks.
By 2025, 70% of the top 20 biologic drugs will have biosimilar competition. If we get this right, millions will save money without losing health. If we get it wrong, we’ll create confusion, distrust, and avoidable harm. The technology is ready. The question is: are we?
Can any biosimilar be swapped for the original drug without a doctor’s approval?
No. Only biosimilars that have received the FDA’s official “interchangeable” designation can be substituted automatically by a pharmacist. Most biosimilars on the market are not interchangeable. You must check the FDA’s database or ask your pharmacist to confirm the status of the specific product.
Are interchangeable biosimilars safer or more effective than regular biosimilars?
No. All FDA-approved biosimilars, whether interchangeable or not, meet the same strict standards for safety, purity, and potency as the original biologic. The “interchangeable” label only means the FDA has confirmed that switching between the original and the biosimilar multiple times won’t harm you. It does not mean the drug is better.
Why do some states require prescriber approval for substitution?
Some states require prescriber approval because of concerns about patient safety, especially for complex conditions like rheumatoid arthritis or psoriasis. Studies have shown that non-medical switching can lead to higher discontinuation rates in some patients. These states prioritize doctor-patient continuity over cost savings, especially when the patient is stable on their current treatment.
Can I be switched from one biosimilar to another without my doctor knowing?
No. The FDA’s interchangeability designation only applies to switching between the reference biologic and a specific interchangeable biosimilar. You cannot automatically switch from one biosimilar to another-like from Cyltezo to Hyrimoz-without your doctor’s approval. Each biosimilar is treated as a separate product, even if they’re copies of the same original drug.
What should I do if I think my biosimilar is causing side effects?
Contact your doctor right away. Keep the medication bottle and note the manufacturer name. Side effects may be caused by the active ingredient or by inactive ingredients (excipients) unique to that biosimilar. Report the issue to your pharmacist and ask if you were switched. You have the right to return to your original medication if needed.
Will my insurance force me to switch to a biosimilar?
Many insurance plans require you to try a biosimilar first if one is available and interchangeable. This is called “step therapy.” But if your doctor writes “dispense as written” on the prescription, the pharmacy must follow it. Always check your plan’s formulary and ask your pharmacist if substitution is mandatory in your case.